
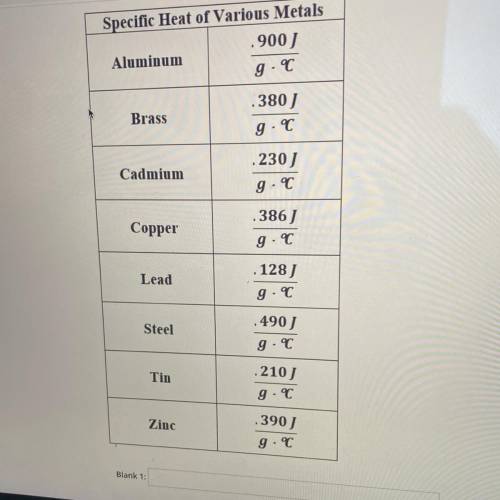
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of water, the water increased temperature from 20.1°C to 35.4°C
If water has a specific heat capacity of 4.18 Jg, determine the unknown metal by calculating it's specific heat. The unknown metal is ___

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 15:00
Which substance is a steroid? cholesterol fatty acid monosaccharide trans fat
Answers: 1

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2
You know the right answer?
WHAT IS THE UNKNOWN METAL?
A 110 g block of metal was heated to 100°C. When transfered to 100 g of...
Questions


Mathematics, 17.10.2019 04:20


French, 17.10.2019 04:20




Mathematics, 17.10.2019 04:20


Social Studies, 17.10.2019 04:20

Social Studies, 17.10.2019 04:20

Mathematics, 17.10.2019 04:20


Mathematics, 17.10.2019 04:20





Social Studies, 17.10.2019 04:20




