
Chemistry, 14.04.2021 18:10 2022mcwhirterbrendan
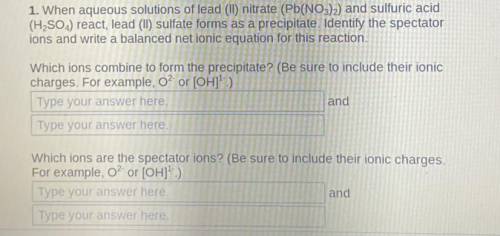
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) react, lead (II) sulfate forms as a precipitate.
Identify the spectator
ions and write a balanced net ionic equation for this reaction.
Which ions combine to form the precipitate? (Be sure to include their ionic
charges. For example, o? or [OH]1- )
Which ions are the spectator ions?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1


Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
You know the right answer?
1. When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid
(H2SO4) react, lead (II...
Questions

English, 27.04.2021 15:40







English, 27.04.2021 15:40


History, 27.04.2021 15:40


Mathematics, 27.04.2021 15:40


Mathematics, 27.04.2021 15:40

Mathematics, 27.04.2021 15:40




Chemistry, 27.04.2021 15:40




