Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:00
6. using 3 – 4 sentences explain (in your own words) why water expands when it freezes? 7. using your knowledge of colligative properties explain whether sodium chloride or calcium chloride would be a more effective substance to melt the ice on a slick sidewalk. use 3 – 4 sentences in your explanation.
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
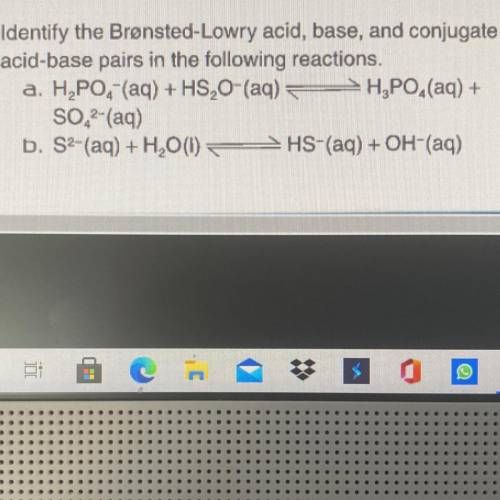
Identify the Bronsted-Lowry Acid, base, and conjugate acid-base pairs in the following reactions. H2...
Questions

English, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01


Mathematics, 11.10.2020 23:01


English, 11.10.2020 23:01

Social Studies, 11.10.2020 23:01




History, 11.10.2020 23:01

English, 11.10.2020 23:01



English, 11.10.2020 23:01

Mathematics, 11.10.2020 23:01


Biology, 11.10.2020 23:01