
Chemistry, 14.04.2021 17:50 MrKrinkle77
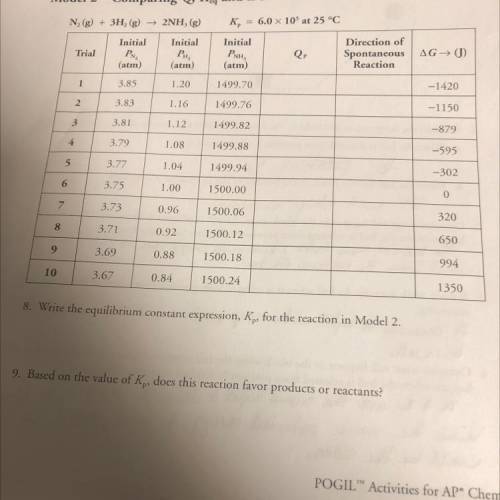
8. Write the equilibrium constant expression, Kp, for the reaction in Model 2. 9. Based on the value of Kp, does this reaction favor products or reactants?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 22.06.2019 10:30
Which characteristics can be used to differentiate star systems? check all that apply.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1

Chemistry, 22.06.2019 22:00
Choose all the answers that apply. fluorine (f) has an atomic number of 9 and an atomic weight of 18.99. fluorine has a. 9 protons b. 10 neutrons c. 18 electrons d. an atomic mass of 19 e. at least one isotope
Answers: 1
You know the right answer?
8. Write the equilibrium constant expression, Kp, for the reaction in Model 2.
9. Based on the valu...
Questions


Social Studies, 21.11.2020 03:50


History, 21.11.2020 03:50

Mathematics, 21.11.2020 03:50


Social Studies, 21.11.2020 03:50

World Languages, 21.11.2020 03:50

Social Studies, 21.11.2020 03:50

Mathematics, 21.11.2020 03:50

Physics, 21.11.2020 03:50



Mathematics, 21.11.2020 03:50



Mathematics, 21.11.2020 03:50



Mathematics, 21.11.2020 03:50



