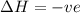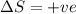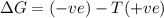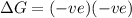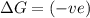Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
1. calculate the approximate enthalpy of the reaction in joules. estimate that 1.0 ml of vinegar has the same thermal mass as 1.0 ml of water. iqnore the thermal mass of th sodium bicarbonate. note: it takes about 4.2 joules () to change 1.0 gram (1.0ml) of water 1.0 c
Answers: 2

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3


Chemistry, 23.06.2019 01:00
Iron (fe) reacts with copper sulfate (cuso4) to form iron (ii) sulfate. in this reaction, cu2+ gains electrons to form cu. which statement is true about this reaction? fe(s) + cuso4(aq) → feso4(aq) + cu(s)
Answers: 3
You know the right answer?
What does a process require to be spontaneous at all temperatures? answer a catalyst and lower acti...
Questions


English, 21.05.2021 22:30



Mathematics, 21.05.2021 22:30

History, 21.05.2021 22:30

English, 21.05.2021 22:30








History, 21.05.2021 22:30


Spanish, 21.05.2021 22:30

Chemistry, 21.05.2021 22:30

Mathematics, 21.05.2021 22:30

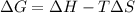
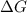 = free energy change
= free energy change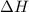 = enthalpy change
= enthalpy change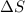 = entropy change
= entropy change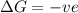 , reaction is spontaneous
, reaction is spontaneous