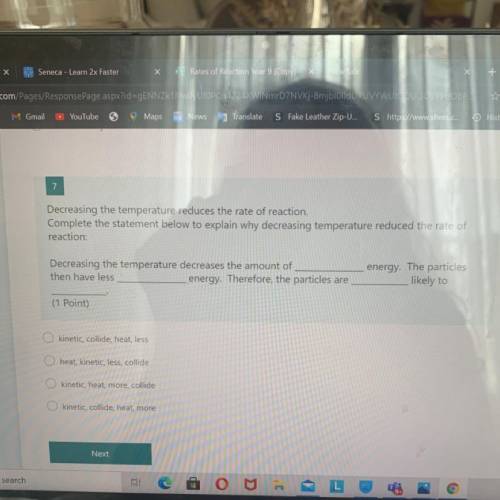
7
Decreasing the temperature reduces the rate of reaction.
Complete the statement below to ex...

Chemistry, 14.04.2021 08:10 jillyan8233
7
Decreasing the temperature reduces the rate of reaction.
Complete the statement below to explain why decreasing temperature reduced the rate of
reaction:
Decreasing the temperature decreases the amount of
then have less
energy. Therefore, the particles are
energy. The particles
likely to
(1 Point)
kinetic, collide, heat, less
heat, kinetic, less, collide
kinetic, heat, more, collide
O kinetic, collide, heat, more
Next

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
When an object falls through the air and encounters air resistance its overall speed will be than if it had not encountered air resistance? (one word answer)
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
Questions

Computers and Technology, 24.10.2019 00:00


Spanish, 24.10.2019 00:00

History, 24.10.2019 00:00

Chemistry, 24.10.2019 00:00


Chemistry, 24.10.2019 00:00


History, 24.10.2019 00:00

Mathematics, 24.10.2019 00:00



Mathematics, 24.10.2019 00:00

History, 24.10.2019 00:00

History, 24.10.2019 00:00







