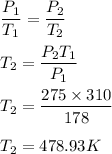Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1


Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
The pressure in a car tire is 178 kPa at 37°C. After a long drive, the pressure is 275 kPa. What is...
Questions





Arts, 04.05.2020 22:33

Social Studies, 04.05.2020 22:33

History, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33



Mathematics, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33

Biology, 04.05.2020 22:33

History, 04.05.2020 22:33


Biology, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33

Mathematics, 04.05.2020 22:33