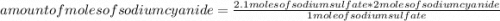Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 05:50
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
How many moles of sodium cyanide (NaCN) would be needed to produce
2.1 moles of sodium sulfate (Na2...
Questions

Mathematics, 22.05.2020 05:06


Computers and Technology, 22.05.2020 05:06



Mathematics, 22.05.2020 05:06


Mathematics, 22.05.2020 05:06


Computers and Technology, 22.05.2020 05:06




History, 22.05.2020 05:06

History, 22.05.2020 05:06


Social Studies, 22.05.2020 05:06