
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of
sodium azide, NaN3.
2 NaNz (s) 2 Na (s) + 3 N2 (g)
If an air bag has a volume of 53.4 L and is to be filled with nitrogen gas at a pressure of 1.07 atm and a
temperature of 23.7°C, how many moles of NaNz must decompose? You may assume the Ny behaves as
an ideal gas.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:20
One or more substances changing into one or more substances is an example of a
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 14:00
The two naturally occurring isotopes of chlorine are 35cl (34.969 amu, 75.77%) and 37cl (36.966 amu, 24.23%). the two naturally occurring isotopes of bromine are 79br (78.918 rm amu, 50.69%) and 81br (80.916 amu, 49.31%). chlorine and bromine combine to form bromine monochloride, brcl. 1. how many peaks will be present in a mass spectrum for brcl? the four combinations of molecule possible given these four isotopes are: 81br37cl, 81br35cl, 79br37cl, and 79br35cl. 2. what are the masses of the four different brcl molecules? express the masses using six significant figures, in decreasing numeric order (highest to lowest), separated by commas.
Answers: 3

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions

Mathematics, 24.06.2021 14:00


English, 24.06.2021 14:00


English, 24.06.2021 14:00

Mathematics, 24.06.2021 14:00

Mathematics, 24.06.2021 14:00


English, 24.06.2021 14:00

History, 24.06.2021 14:00


Mathematics, 24.06.2021 14:00

Mathematics, 24.06.2021 14:00



Mathematics, 24.06.2021 14:00

Physics, 24.06.2021 14:00

History, 24.06.2021 14:00


Advanced Placement (AP), 24.06.2021 14:00

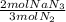 = 1.57 mol NaN₃
= 1.57 mol NaN₃

