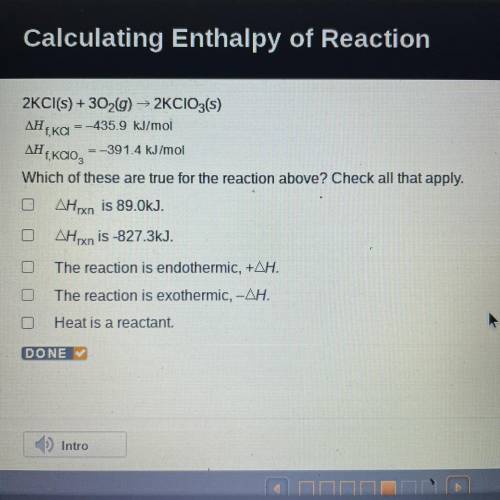
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,<...

Chemistry, 13.04.2021 07:10 Harini5721
2KCl(s) + 3029) ► 2KCIO3(s)
ΔΗ, = -435.9 kJ/mol
f, KCI
ΔΗ
=-391.4 kJ/mol
,
Which of these are true for the reaction above? Check all that apply.
AHxn is 89.0kJ.
OAHrxn is -827.3kJ.
The reaction is endothermic, +AH.
O The reaction is exothermic, -AH.
Heat is a reactant.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
The compound methyl butanoate smells like apples. its percent composition is 58.8% c, 9.9% h, and 31.4% o. what’s the empirical formula ?
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
Questions

Chemistry, 05.11.2020 02:40

Engineering, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40


History, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40

Biology, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40





Biology, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40

Mathematics, 05.11.2020 02:40




