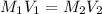Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The image shows a process that releases nuclear energy which statement best identifies the process shown the process must be fusion because energy is released the process must be fusion because of have your nucleus formed a smaller nuclei the process must be fission because a large nucleus breaks into smaller nuclei the process must be fission because neutrons are formed
Answers: 1

Chemistry, 22.06.2019 15:00
Many ionic compounds and a few highly polar covalent compounds are because they completely ionize in water to create a solution filled with charged ions that can conduct an electric current.
Answers: 1

Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

You know the right answer?
Mr. Whiteman has two stock solutions of sulfuric acid (H2SO4) in the supply closet: 12.1 M and 6.0 M...
Questions


Mathematics, 19.04.2021 18:00




Mathematics, 19.04.2021 18:00


Mathematics, 19.04.2021 18:00


English, 19.04.2021 18:00




History, 19.04.2021 18:00

Chemistry, 19.04.2021 18:00

Biology, 19.04.2021 18:00


English, 19.04.2021 18:00

Mathematics, 19.04.2021 18:00