
Chemistry, 13.04.2021 01:30 mendezmarco2004
A 10.0-mL sample of sulfuric acid, H2SO4, from a battery of an old car is diluted to 100.0 mL, and a 10.00-mL aliquot (portion) of the diluted acid is then titrated with 0.2500 M NaOH solution. If the concentration of H2SO4 in the original battery was 3.25 M, how many milliliters (mL) of the NaOH solution is required to titrate the sulfuric acid present in the 10.0-mL portion of dilute acid solution

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2
You know the right answer?
A 10.0-mL sample of sulfuric acid, H2SO4, from a battery of an old car is diluted to 100.0 mL, and a...
Questions



Mathematics, 14.04.2021 03:20

Law, 14.04.2021 03:20

Mathematics, 14.04.2021 03:20

History, 14.04.2021 03:20

Mathematics, 14.04.2021 03:20



Physics, 14.04.2021 03:20




Arts, 14.04.2021 03:20



Mathematics, 14.04.2021 03:20

Mathematics, 14.04.2021 03:20


Mathematics, 14.04.2021 03:20

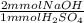 = 6.5 mmol NaOH
= 6.5 mmol NaOH

