
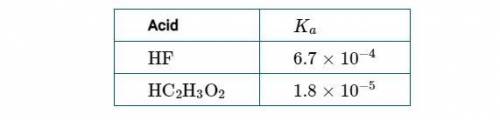
Chemistry, 12.04.2021 22:00 macylen3900
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K for the process represented by the equation H2O(l)⇄H2O(g).
(b) Considering your answer to part (a), indicate whether the process is thermodynamically favorable at 298K. Justify your answer.
(c) Considering your answer to part (b), explain why H2O(l) has a measurable equilibrium vapor pressure at 298K.
(2nd Screenshot)
Water vapor can be produced in two different processes, as represented below.
(d) In terms of concepts of entropy and the particle-level structure of the different phases of water, explain why the change in entropy, ΔS, is greater for process 1 than for process 2.
Please help as soon as possible


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3


Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
(1st Screenshot)
(a) Using the information in the table above, determine the value of ΔG° at 298K f...
Questions


Chemistry, 31.07.2019 15:30


Biology, 31.07.2019 15:30





Biology, 31.07.2019 15:30

Biology, 31.07.2019 15:30

Biology, 31.07.2019 15:30

Social Studies, 31.07.2019 15:30

Social Studies, 31.07.2019 15:30

Social Studies, 31.07.2019 15:30



Business, 31.07.2019 15:30


Social Studies, 31.07.2019 15:30



