
Chemistry, 12.04.2021 21:50 twiddleturd
In this experiment, you will have a chance to test the hypothesis that Ernest Rutherford used when determining the size of the nucleus. In his "gold foil experiment," Rutherford shot alpha particles at gold atoms. Once he realized that the alpha particles were hitting a concentrated positive mass, he developed the nuclear model of the atom. Next, he set out to determine the relative size of the nucleus compared to the rest of the atom. He reasoned that the smaller the nucleus, the less likely it was to be hit by an alpha particle. This led to a simple comparative ratio:
It took a great number of shots to actually hit the nucleus because the size of the atom was so much larger than the nucleus. Rutherford proposed that the "hit ratio" was approximately equal to the volume ratio. This is the hypothesis you will test in this experiment.
OBJECTIVES
Investigate a scientific hypothesis.
Present your findings in a scientific report.
Online Lab
The animation will help you test Rutherford’s hypothesis. Be sure to record the dimensions of the box and the block so that you can find their volumes when you present your findings.
00:4001:33
SHOW TRANSCRIPT
Present Your Findings
When you are finished with the experiment, complete the following data analysis and record your answers in the Essay box below.
Determine the volume of the box and the block.
Determine the ratio of the block to the box:
Multiply this number by 100 to turn it into a percent.
Complete this statement: The volume of the block is _ percent of the volume of the box.
Determine the ratio of the number of hits to the number of shots:
Multiply this number by 100 to turn it into a percent.
Complete this statement: The block was hit _ percent of the time.
Compare the results of step 2 to the results of step 3. Are the percentages similar?
Write a conclusion discussing the following items:
Based on your findings, do you think Rutherford's hypothesis was reasonable?
Restate Rutherford's hypothesis and describe how you tested it.
State whether your results support the hypothesis. If they do not, can you suggest some error in experimental procedure (other than general human error) that might explain it?
Finally, explain how this experiment confirms the nuclear model of the atom and the idea that most of the atom is empty space.
Length of box 20.75 in.
Width of box 14.25 in.
Height of box 12 in.
Length of block 2.5 in.
Width of block 1 in.
Height of block 1 in.
Number of hits on the block 2
Total number of shots 100
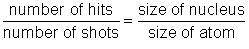
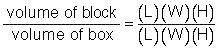


Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

You know the right answer?
In this experiment, you will have a chance to test the hypothesis that Ernest Rutherford used when d...
Questions



Mathematics, 14.02.2022 04:20

Social Studies, 14.02.2022 04:20

Mathematics, 14.02.2022 04:20

Social Studies, 14.02.2022 04:30


English, 14.02.2022 04:30

Mathematics, 14.02.2022 04:30




Social Studies, 14.02.2022 04:30


Mathematics, 14.02.2022 04:30


Medicine, 14.02.2022 04:30

Mathematics, 14.02.2022 04:30

English, 14.02.2022 04:30

Computers and Technology, 14.02.2022 04:30



