
Chemistry, 12.04.2021 18:10 jameskarbar9p8c9d2
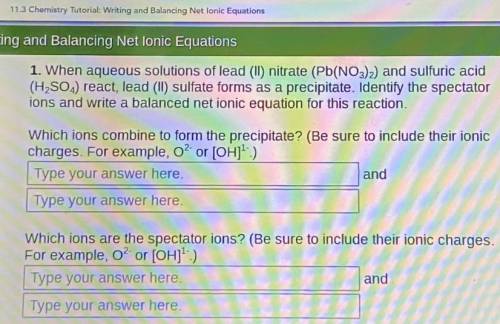
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II) sulfate forms as a precipitate. Identify the spectator ions and write a balanced net ionic equation for this reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

You know the right answer?
When aqueous solutions of lead (II) nitrate (Pb(NO3)2) and sulfuric acid (H, SO.,) react, lead (II)...
Questions



Mathematics, 25.02.2021 20:30

Mathematics, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30


Mathematics, 25.02.2021 20:30


Advanced Placement (AP), 25.02.2021 20:30

Mathematics, 25.02.2021 20:30


Chemistry, 25.02.2021 20:30





English, 25.02.2021 20:30

Mathematics, 25.02.2021 20:30




