
Chemistry, 12.04.2021 08:20 shadoris26
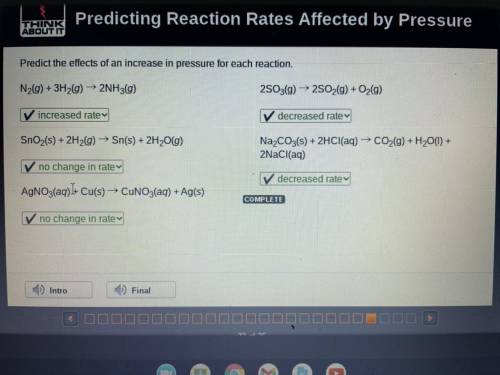
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g) ► 2S02(g) + O2(g)
SnO2(s) + 2H2(g) → Sn(s) + 2H20(g)
Na2CO3(s) + 2HCl(aq) → CO2(g) + H20(1) +
2NaCl(aq)
AgNO3(aq))+ Cu(s) → CUNO3(aq) + Ag(s)
COMPLETE

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1
You know the right answer?
Predict the effects of an increase in pressure for each reaction.
N2(g) + 3H2(g) → 2NH3(g) 2S03(g)...
Questions



Health, 14.07.2019 06:00

Mathematics, 14.07.2019 06:00

Biology, 14.07.2019 06:00

Mathematics, 14.07.2019 06:00




Mathematics, 14.07.2019 06:00

History, 14.07.2019 06:00

Mathematics, 14.07.2019 06:00


Mathematics, 14.07.2019 06:00

Health, 14.07.2019 06:00


English, 14.07.2019 06:00


Mathematics, 14.07.2019 06:00

Chemistry, 14.07.2019 06:00



