
Chemistry, 21.10.2019 17:40 allieb12334
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation below, how many grams of chromium oxide can be formed? show all your work for the calculations for full credit.
4cr + 3o2 yields 2cr2o3

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Transportation is the largest single source of air pollution in the united states. air pollution can harm the environment and human health. which technology could offer a solution to this problem? mufflers that reduce noise motors that run on electricity tires that improve gas mileage
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1

Chemistry, 23.06.2019 00:30
Arrange the elements in order of increasing electronegativity. use the periodic table to you arrange the elements. p o k mg
Answers: 2
You know the right answer?
If 143 grams of chromium react with an excess of oxygen, as shown in the balanced chemical equation...
Questions

Computers and Technology, 29.11.2020 09:30


Mathematics, 29.11.2020 09:30


English, 29.11.2020 09:30

Business, 29.11.2020 09:30


Mathematics, 29.11.2020 09:30

Mathematics, 29.11.2020 09:30


English, 29.11.2020 09:30


Social Studies, 29.11.2020 09:30

Mathematics, 29.11.2020 09:30



Mathematics, 29.11.2020 09:30


Mathematics, 29.11.2020 09:30

English, 29.11.2020 09:30

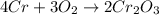
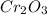 = 152g/mol
= 152g/mol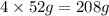 of Chromium produces
of Chromium produces 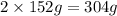 of
of  of Chromium oxide(
of Chromium oxide(

