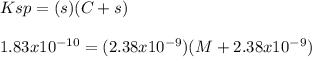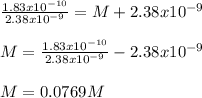Chemistry, 11.04.2021 23:20 xeskimopie
What is the molarity of NaCl in which AgCl has a molar solubility of 2.38 x 10-9 mol /L? The Ksp for Silver Chloride is: 1.83 x 10-10.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Can smoke be transformed into liquid or used as energy or both?
Answers: 2

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
What is the molarity of NaCl in which AgCl has a molar solubility of 2.38 x 10-9 mol /L? The Ksp for...
Questions

Mathematics, 14.05.2021 16:40

Mathematics, 14.05.2021 16:40





History, 14.05.2021 16:40


History, 14.05.2021 16:40



Mathematics, 14.05.2021 16:40

Mathematics, 14.05.2021 16:40




Mathematics, 14.05.2021 16:40

Biology, 14.05.2021 16:40

Mathematics, 14.05.2021 16:40

![AgCl(s)\rightleftharpoons Ag^+(aq)+Cl^-(aq)\\\\Ksp=[Ag^+][Cl^-]\\](/tpl/images/1251/6223/6391c.png)