
Chemistry, 11.04.2021 22:20 andreagrimaldo4
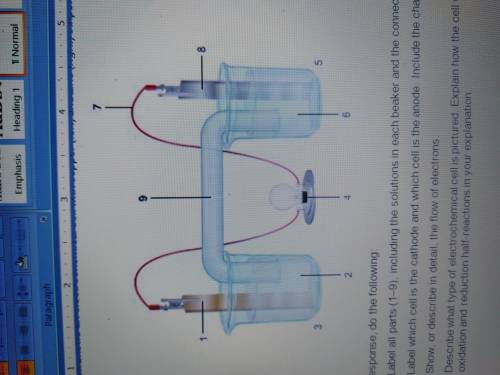
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
In your response, do the following:
• Label all parts (1–9), including the solutions in each beaker and the connecting tube.
• Label which cell is the cathode and which cell is the anode. Include the charge on each strip.
• Show, or describe in detail, the flow of electrons.
• Describe what type of electrochemical cell is pictured. Explain how the cell works. Include the oxidation and reduction half-reactions in your explanation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of sodium-23 (atomic number = 11) has a positive charge of +1. give this information, how many electrons does it have? how many proteins and neutrons does this atom have
Answers: 2

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1
You know the right answer?
1. The diagram shows an electrochemical cell with copper (left) and zinc (right) strips.
In your re...
Questions

Social Studies, 17.08.2020 17:01


Mathematics, 17.08.2020 17:01

History, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01



Mathematics, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01




Mathematics, 17.08.2020 17:01

History, 17.08.2020 17:01

History, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Arts, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01

Mathematics, 17.08.2020 17:01




