
Chemistry, 11.04.2021 20:10 michaelhal4271
(c) Write the balanced neutralization reaction and calculate the volume in mL of a 1.420 M NaOH solution required to titrate 25.00 mL of a 5.400 M H2SO4 solution

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
(c) Write the balanced neutralization reaction and calculate the volume in mL of a 1.420 M NaOH solu...
Questions

Mathematics, 15.12.2020 18:30

History, 15.12.2020 18:30


Computers and Technology, 15.12.2020 18:30

Mathematics, 15.12.2020 18:30

Mathematics, 15.12.2020 18:30



Physics, 15.12.2020 18:30

Mathematics, 15.12.2020 18:30







Mathematics, 15.12.2020 18:30



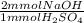 = 270 mmol NaOH
= 270 mmol NaOH

