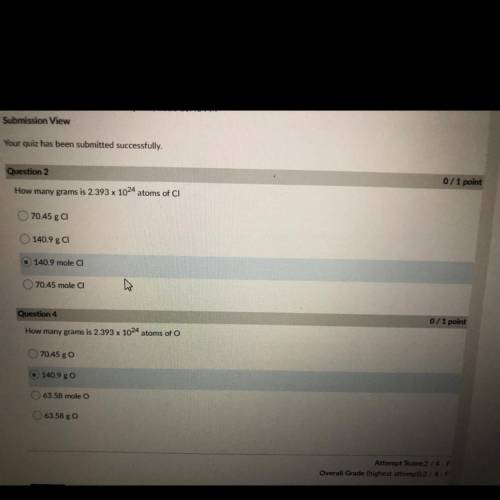
How many grams is 2.393 x 10^24 atoms of CI
70.45 g CI
140.9 ga
140.9 mole CI
70....


Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1
You know the right answer?
Questions


History, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50


English, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50


Mathematics, 21.04.2021 01:50




Social Studies, 21.04.2021 01:50