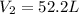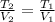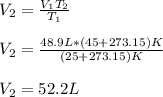Chemistry, 11.04.2021 05:00 madiforkner
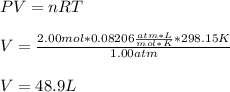
A gas system contains 2.00 moles of O2 and CO2 gas, has an initial temperature of 25.0 oC and is under 1.00 atm of pressure. If the pressure remains constant and the temperature is raised to 45.0 oC, then what will the new volume (assuming a closed system) be?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2
You know the right answer?
A gas system contains 2.00 moles of O2 and CO2 gas, has an initial temperature of 25.0 oC and is und...
Questions

Mathematics, 03.12.2021 01:40

History, 03.12.2021 01:40



History, 03.12.2021 01:40


Computers and Technology, 03.12.2021 01:40


Computers and Technology, 03.12.2021 01:40

Computers and Technology, 03.12.2021 01:40



Mathematics, 03.12.2021 01:40



Mathematics, 03.12.2021 01:40

Computers and Technology, 03.12.2021 01:40


English, 03.12.2021 01:40