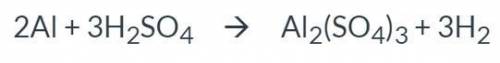Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 09:40
Apiece of copper has a temperature of 75.6 0c. when the metal is placed in 100.0 grams of water at 19.1 0c, the temperature rises by 5.5 0c. what is the mass of the metal?
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
Calculate the number of moles of H2SO4 required to react with 18.79 moles of Al.
please do step by...
Questions

Computers and Technology, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30


Business, 02.10.2019 17:30


Mathematics, 02.10.2019 17:30


Mathematics, 02.10.2019 17:30


English, 02.10.2019 17:30

Physics, 02.10.2019 17:30



Business, 02.10.2019 17:30

Mathematics, 02.10.2019 17:30



Mathematics, 02.10.2019 17:30