Calculate the pH for the following weak acids
.01M Formic acid (HCHO2) aka= 1.7 x 10^-4...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1


Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 22:00
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
You know the right answer?
Questions

Chemistry, 15.10.2020 18:01



Biology, 15.10.2020 18:01



Mathematics, 15.10.2020 18:01

English, 15.10.2020 18:01




English, 15.10.2020 18:01

Biology, 15.10.2020 18:01


Mathematics, 15.10.2020 18:01



Biology, 15.10.2020 18:01

Computers and Technology, 15.10.2020 18:01

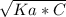 pH = -log[H⁺]
pH = -log[H⁺]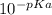 and C is the molar concentration.
and C is the molar concentration.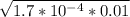 [H⁺] = 0.0013 M
[H⁺] = 0.0013 M

