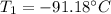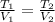Chemistry, 10.04.2021 03:50 ehgdhjahag
An ideal gas in a sealed container has an initial volume of 2.80 L. At constant pressure, it is cooled to 18.00 °C, where its
final volume is 1.75 L. What was the initial temperature?
Ti =
'c

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 23.06.2019 00:30
If there are 3.5 moles of koh, how many moles of naoh can be produced? question 1 options: a)3.0 moles naoh b)3.5 moles naoh c)1 moles naoh d)9 moles naoh
Answers: 1

Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 02:00
Scientists are often interested in knowing the molar heat of combustion – the heat released during the combustion of one mole of a substance. use the periodic table to find molar masses. how many moles of ethanol are present in the sample?
Answers: 2
You know the right answer?
An ideal gas in a sealed container has an initial volume of 2.80 L. At constant pressure, it is cool...
Questions

English, 05.02.2022 14:00



English, 05.02.2022 14:00

English, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00



Business, 05.02.2022 14:00


Biology, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00



Social Studies, 05.02.2022 14:00

Business, 05.02.2022 14:00

Geography, 05.02.2022 14:00

Mathematics, 05.02.2022 14:00