
Chemistry, 08.04.2021 22:20 cornpops4037
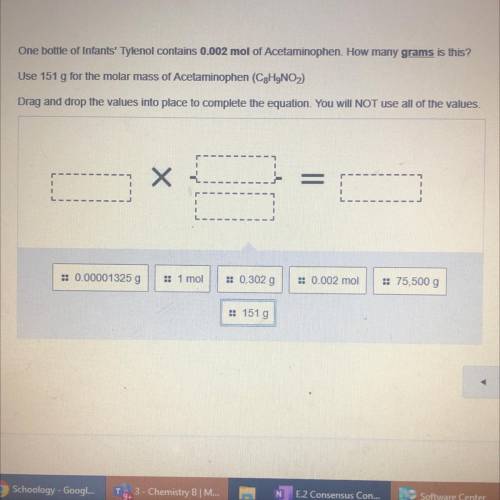
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151 g for the molar mass of Acetaminophen (C2H9NO)
Drag and drop the values into place to complete the equation. You will NOT use all of the values.
Х
= 0.00001325 g
1 moi
:: 0.302 g
: 0.002 mol
= 75,500 g
:: 151 g

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
The volume of helium in a blimp is 6.28 x 10^9 millimeters. the density of helium in the blimp is .1786 kilogram/meter^3. find the mass of the helium in the blimp.
Answers: 1

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1
You know the right answer?
One bottle of Infants' Tylenol contains 0.002 mol of Acetaminophen. How many grams is this?
Use 151...
Questions




History, 02.10.2019 19:50


Mathematics, 02.10.2019 19:50

Chemistry, 02.10.2019 19:50


Mathematics, 02.10.2019 19:50

Chemistry, 02.10.2019 19:50


History, 02.10.2019 19:50



Mathematics, 02.10.2019 19:50


Biology, 02.10.2019 19:50

Mathematics, 02.10.2019 19:50

Health, 02.10.2019 19:50



