
A 5.0 g sample of a pesticide was decomposed with metallic sodium in alcohol, and the liberated chloride ion was precipitated with AgCl. Express the results of this analysis in terms of percent DDT (C14H9Cl5, molar mass, 354.72 g/mol)) based on the recovery of 0.1606 g of AgCl

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:30
When a scientific theory has been tested and proved by the scientific community, it becomes a law
Answers: 2

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2
You know the right answer?
A 5.0 g sample of a pesticide was decomposed with metallic sodium in alcohol, and the liberated chlo...
Questions


English, 08.04.2021 16:30


Computers and Technology, 08.04.2021 16:30



Physics, 08.04.2021 16:30

Mathematics, 08.04.2021 16:30

English, 08.04.2021 16:30


Mathematics, 08.04.2021 16:30

Social Studies, 08.04.2021 16:30

Mathematics, 08.04.2021 16:30





Mathematics, 08.04.2021 16:30


Law, 08.04.2021 16:30

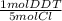 = 2.24x10⁻⁴ mol DDT
= 2.24x10⁻⁴ mol DDT

