
Chemistry, 08.04.2021 08:30 sharkboy3780
A 10.0 mL sample of H2SO4 was exactly neutralized by 23.5 mL of 1.00 M KOH. Calculate the molarity of the acid. H2SO4(aq) + KOH(aq) —> K2SO4(aq) + H2O(l)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
If 34.2 grams of lithium react with excess water, how many liters of hydrogen gas can be produced at 299 kelvin and 1.21 atmospheres? 2 li (s) + 2 h2o (l) yields 2 lioh (aq) + h2 (g)
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1
You know the right answer?
A 10.0 mL sample of H2SO4 was exactly neutralized by 23.5 mL of 1.00 M KOH. Calculate the molarity o...
Questions

Mathematics, 04.04.2021 18:30

English, 04.04.2021 18:30






Chemistry, 04.04.2021 18:30


Mathematics, 04.04.2021 18:30

History, 04.04.2021 18:30

Mathematics, 04.04.2021 18:30



Mathematics, 04.04.2021 18:30

Computers and Technology, 04.04.2021 18:30

Business, 04.04.2021 18:30



Computers and Technology, 04.04.2021 18:30

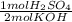 = 11.75 mmol H₂SO₄
= 11.75 mmol H₂SO₄

