
Chemistry, 07.04.2021 19:50 nyasiasaunders1234
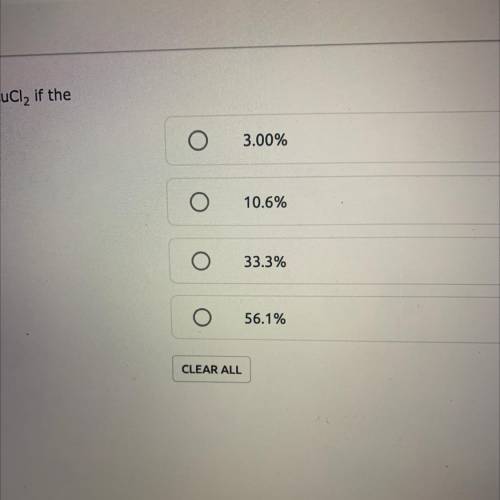
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield is 18.81g? % Yield = (Actual Yield/Theoretical Yield) x 100

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Y=‐1x + 7 if y has a value of ‐24 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 00:30
Water (2510 g ) is heated until it just begins to boil. if the water absorbs 5.09×105 j of heat in the process, what was the initial temperature of the water?
Answers: 3

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 01:30
There are main groups in the modern periodic table of elements
Answers: 1
You know the right answer?
A reaction yields 6.26 grams of a CuCl2. What is the percent yield of CuCl2 if the theoretical yield...
Questions


Mathematics, 18.09.2021 03:50



Mathematics, 18.09.2021 03:50

Physics, 18.09.2021 03:50


Mathematics, 18.09.2021 03:50

Mathematics, 18.09.2021 03:50




Mathematics, 18.09.2021 03:50


Mathematics, 18.09.2021 03:50

Mathematics, 18.09.2021 03:50

Mathematics, 18.09.2021 03:50


Mathematics, 18.09.2021 03:50

Mathematics, 18.09.2021 03:50



