Chemistry, 06.04.2021 17:20 momzsons4834
Will mark Brainliest
In an experiment, hydrochloric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each flask became gradually invisible due the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown.
EXPERIMENTAL RECORD
flask vol of HCI vol of sodium sulfate vol of water time
1 10 mL 10 mL 40 mL 14 seconds
2 10 mL 20 mL 30 mL
3 10 mL 30 mL 20 mL
4 10 mL 40 mL 10 mL
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Classify each statement about effective nuclear charge, zeff, as true or false.
Answers: 2

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 22:30
Hot air balloons float in the air because of the difference in density between cold and hot air. in this problem, you will estimate the minimum temperature the gas inside the balloon needs to be, for it to take off. to do this, use the following variables and make these assumptions: the combined weight of the pilot basket together with that of the balloon fabric and other equipment is w. the volume of the hot air inside the balloon when it is inflated is v. the absolute temperature of the hot air at the bottom of the balloon is th (where th> tc). the absolute temperature of the cold air outside the balloon is tc and its density is ďc. the balloon is open at the bottom, so that the pressure inside and outside the balloon is the same. as always, treat air as an ideal gas. use g for the magnitude of the acceleration due to gravity.
Answers: 1

You know the right answer?
Will mark Brainliest
In an experiment, hydrochloric acid reacted with different volumes of sodium t...
Questions


Chemistry, 27.03.2020 19:29



Mathematics, 27.03.2020 19:29

Mathematics, 27.03.2020 19:29

Mathematics, 27.03.2020 19:29




Social Studies, 27.03.2020 19:29

Chemistry, 27.03.2020 19:29

Health, 27.03.2020 19:29

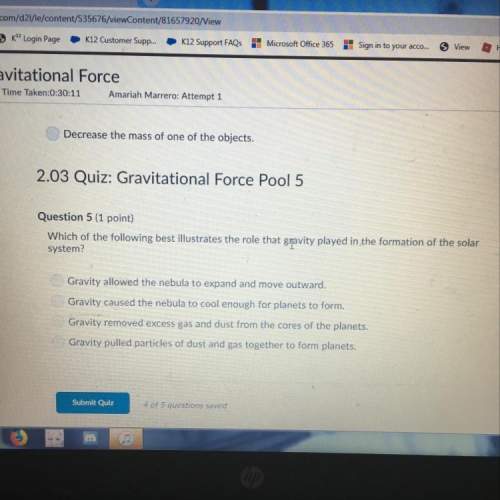
Chemistry, 27.03.2020 19:29





Mathematics, 27.03.2020 19:29

Chemistry, 27.03.2020 19:29