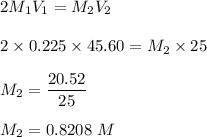Chemistry, 06.04.2021 15:10 wcwheelsp26w7n
It takes 45.60 mL of a 0.225 M hydrochloric acid solution to react completely with 25.00 mL of calcium hydroxide in this reaction below, what is the molar concentration of the calcium hydroxide solution? 2HCl(aq) + Ca(OH)2(aq)!CaCl2(aq) + 2H2O(l)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

Chemistry, 22.06.2019 13:30
Some animals that try to adapt to climate changes eventually die due to starvation, as climate change alters the web.
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
It takes 45.60 mL of a 0.225 M hydrochloric acid solution to react completely with 25.00 mL of calci...
Questions



Social Studies, 14.12.2020 05:40

Mathematics, 14.12.2020 05:40


English, 14.12.2020 05:40






English, 14.12.2020 05:40

Chemistry, 14.12.2020 05:40


Mathematics, 14.12.2020 05:40

Computers and Technology, 14.12.2020 05:40

Mathematics, 14.12.2020 05:40


Mathematics, 14.12.2020 05:40