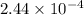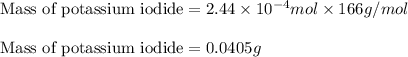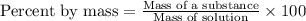Chemistry, 06.04.2021 05:10 jjimenez0276
The iodide in a sample that also contained chloride was converted to iodate by treatment with an excess of bromine: The unused bromine was removed by boiling; an excess of barium ion was then added to precipitate the iodate: In the analysis of a 1.54-g sample, 0.0596 g of barium iodate was recovered. Express the results of this analysis as percent potassium iodide.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 23.06.2019 00:00
Total the mass on the syringe. record it in the correct row of the data table. kg done click and drag weights to change the pressure. click the syringe to zoom in and see the volume. intro
Answers: 3

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 03:00
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3
You know the right answer?
The iodide in a sample that also contained chloride was converted to iodate by treatment with an exc...
Questions



Computers and Technology, 29.01.2021 23:40


Mathematics, 29.01.2021 23:40

English, 29.01.2021 23:40


English, 29.01.2021 23:40


Mathematics, 29.01.2021 23:40



Mathematics, 29.01.2021 23:40

Mathematics, 29.01.2021 23:40

Mathematics, 29.01.2021 23:40

Mathematics, 29.01.2021 23:40

English, 29.01.2021 23:40

Social Studies, 29.01.2021 23:40


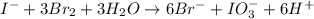 (i)
(i) ......(ii)
......(ii)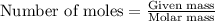
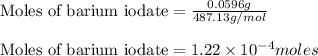
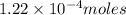 of barium iodate will be produced by
of barium iodate will be produced by 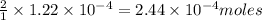 of iodate ions
of iodate ions of iodate ions will be produced by
of iodate ions will be produced by 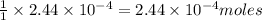 of iodine ions
of iodine ions