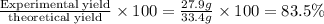Chemistry, 06.04.2021 02:40 naomicervero
If 50.0 g of silicon dioxide is heated with an excess of carbon, 27.9 g of silicon carbide is produced. What is the percent yield of the reaction

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2


Chemistry, 23.06.2019 18:40
Select the correct answer from each drop-down menu. in a reversible reaction, the forward reaction takes place the reverse reaction. such a reaction, on reaching equilibrium, will have .
Answers: 2
You know the right answer?
If 50.0 g of silicon dioxide is heated with an excess of carbon, 27.9 g of silicon carbide is produc...
Questions


History, 15.06.2021 20:20


Mathematics, 15.06.2021 20:20

Mathematics, 15.06.2021 20:20








Mathematics, 15.06.2021 20:20


Mathematics, 15.06.2021 20:20

Mathematics, 15.06.2021 20:20

Mathematics, 15.06.2021 20:20

Mathematics, 15.06.2021 20:20

Mathematics, 15.06.2021 20:20

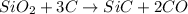
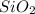 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and 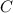 is the excess reagent.
is the excess reagent.
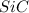
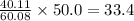 of
of