
Chemistry, 05.04.2021 19:40 softballgirl3589
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0°C, and a pressure of 0.980 atm, and a mass of 0.207 g.
(hint: find moles first and remember that molar mass is the mass per mole”)
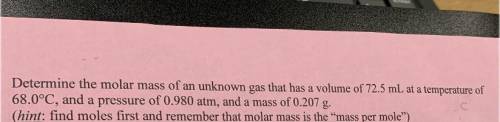

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 23.06.2019 03:30
Which of the following describes the entropy change as a solution is made from a liquid and solid
Answers: 1

Chemistry, 23.06.2019 08:00
Technician a says that you should never jump-start a frozen battery. technician b says that a frozen battery can explode, causing injury, when jump-started. who is correct?
Answers: 2
You know the right answer?
6. Determine the molar mass of an unknown gas that has a volume of 72.5 mL at a temperature of
68.0...
Questions



Mathematics, 23.06.2019 03:30

Chemistry, 23.06.2019 03:30



Mathematics, 23.06.2019 03:30

Mathematics, 23.06.2019 03:30

History, 23.06.2019 03:30

Mathematics, 23.06.2019 03:30


Computers and Technology, 23.06.2019 03:30

English, 23.06.2019 03:30

Mathematics, 23.06.2019 03:30

Mathematics, 23.06.2019 03:30

Health, 23.06.2019 03:30


Mathematics, 23.06.2019 03:30

Mathematics, 23.06.2019 03:30



