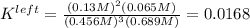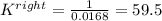Chemistry, 05.04.2021 03:50 addisynshepherd
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine the value of the equilibrium constant if the concentration of product C at equilibrium was measured to be 0.456 M.
Initial Conditions: No reactants present, [C] = 0.651 M, [D] = 0.754 M.
Equilibrium Conditions: [A] = ?, [Y] = ?, [C] = 0.456 M, [D] = ?.
A. K = 59.5
B. K = 37.2
C. K = 0.0269
D. K = 0.0168

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 18:30
When a device is used in a circuit in which the voltage is 81 v the current flowing through the device is 3 a what is the resistance of the device
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2
You know the right answer?
2A (g) + Y (g) <-- --> 3C (g) + D (g)
Based on the initial conditions shown below, determine...
Questions



Mathematics, 30.07.2021 15:30

Computers and Technology, 30.07.2021 15:30


English, 30.07.2021 15:40

Mathematics, 30.07.2021 15:40












English, 30.07.2021 15:40

Mathematics, 30.07.2021 15:40

![K^{left}=\frac{[A]^2[Y]}{[C]^3[D]}](/tpl/images/1239/1292/6d673.png)
![[A]=2x](/tpl/images/1239/1292/d41d8.png)
![[Y]=x](/tpl/images/1239/1292/d76b1.png)
![[C]=0.651M-3x](/tpl/images/1239/1292/474d4.png)
![[D]=0.754M-x](/tpl/images/1239/1292/68d18.png)
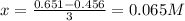
![[A]=2(0.065M)=0.13M](/tpl/images/1239/1292/b6025.png)
![[Y]=0.065M](/tpl/images/1239/1292/afc74.png)
![[D]=0.754M-0.065M=0.689M](/tpl/images/1239/1292/0e883.png)