
Chemistry, 04.04.2021 21:50 rogersdeloris1ovgm3b
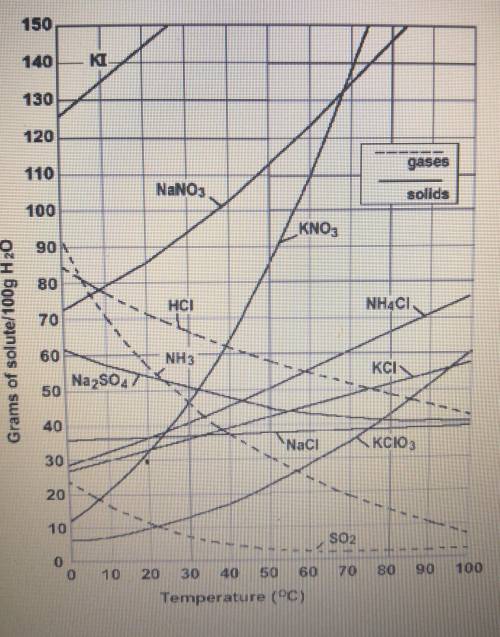
At 50°C, 100g of Sodium nitrate (NaNO3) is dissolved in 100 g of water. Is this solution saturated,
unsaturated, or supersaturated?
O a unsaturated
O b. saturated
O c. Supersaturated

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 01:30
Magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. trial 1: trial 2: data mass of empty crucible with lid trial 1: 26.688 trial 2: 26.681 mass of mg metal, crucible, and lid trial 1: 26.994 trial: 2 26.985 mass of mgo, crucible, and lid trial 1: 27.188 trial 2: 27.180
Answers: 1
You know the right answer?
At 50°C, 100g of Sodium nitrate (NaNO3) is dissolved in 100 g of water. Is this solution saturated,...
Questions



Mathematics, 12.03.2021 07:00


Mathematics, 12.03.2021 07:00

History, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00


Mathematics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

History, 12.03.2021 07:00

History, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

Mathematics, 12.03.2021 07:00

History, 12.03.2021 07:00


Mathematics, 12.03.2021 07:00



