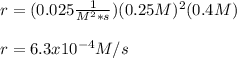Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is...

Study the reaction and read the statement.
Xe+3F2→XeF6
The rate constant for this reaction is 0.025, and the reaction is second order in Xe and first order in F2.
What is the rate of the reaction if [Xe] is 0.25 M, and [F2] is 0.4 M?
4.0 × 10–^4
2.5 × 10–^3
1.5 × 10–^3
6.3 × 10–^4

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 03:00
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2


Chemistry, 23.06.2019 09:30
Sheela and her brother hari were sitting in the living room, watching tv. suddenly hari said that he thinks something is burning in the other room. how did he get the burning smell?
Answers: 3

Chemistry, 23.06.2019 09:50
T(s) in2os] (m) 0 185 2.39 546 1.90 725 1.70 the decomposition of n205 can be described by the equation 2.68 given these data for the reaction at 45°c in carbon tetrachloride solution, calculate the average rate of reaction for each successive time interval. ntr s to 185 s 185 s to 546 s 546 s to 725 s number number number reaction rate: m/s m/s m/s
Answers: 1
You know the right answer?
Questions

Mathematics, 24.03.2021 21:00

Mathematics, 24.03.2021 21:00




Social Studies, 24.03.2021 21:00

Mathematics, 24.03.2021 21:00


Mathematics, 24.03.2021 21:00

Biology, 24.03.2021 21:00



Mathematics, 24.03.2021 21:00



Mathematics, 24.03.2021 21:00




Mathematics, 24.03.2021 21:00

![r=k[Xe]^2[F_2]](/tpl/images/1238/0471/c5483.png)