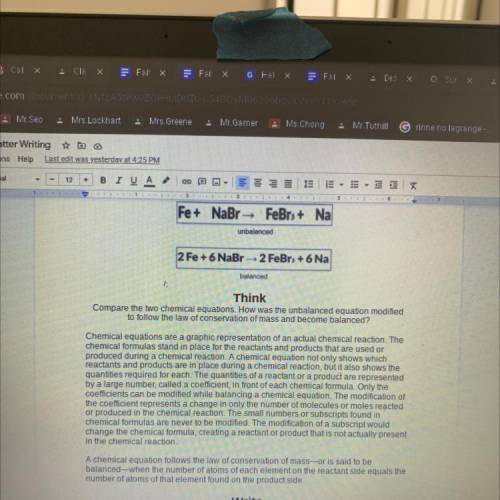
Fe+ NaBr - FeBr3+ Na
unbalanced
2 Fe + 6 NaBr-2 FeBr + 6 Na
Think
Com...

Fe+ NaBr - FeBr3+ Na
unbalanced
2 Fe + 6 NaBr-2 FeBr + 6 Na
Think
Compare the two chemical equations. How was the unbalanced equation modified
to follow the law of conservation of mass and become balanced?
Chemical equations are a graphic representation of an actual chemical reaction. The
chemical formulas stand in place for the reactants and products that are used or
produced during a chemical reaction. A chemical equation not only shows which
reactants and products are in place during a chemical reaction, but it also shows the
quantities required for each. The quantities of a reactant or a product are represented
by a large number called a coefficient in front of each chemical formula. Only the
coefficients can be modified while balancing a chemical equation. The modification of
the coefficient represents a change in only the number of molecules or moles reacted
or produced in the chemical reaction. The small numbers or subscripts found in
chemical formulas are never to be modified. The modification of a subscript would
change the chemical formula, creating a reactant or product that is not actually present
in chemical reaction.
A chemical equation follows the law of conservation of mass-or is said to be
balanced when the number of atoms of each element on the reactant side equals the
number of atoms of that element found on the product side.
Write
• Explain how the chemical reaction above had to be manipulated to follow the
law of conservation of mass,
Why were the large numbers (coefficients) changed but not the small numbers
(subscripts)?
Include the names of the molecules in the products and the reaction
Identify the reaction type
.
NEED HELP BEFORE 4pm PLEASE HELP!!! WILL GIVE BRAINIEST

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:50
Why do scientists look for patterns in the world? a. patterns can explain observations. b. patterns never change, no matter what. c. patterns are easy for scientists to detect. d. patterns are all the same, through all time.
Answers: 1

Chemistry, 22.06.2019 08:30
In the millikan oil drop experiment they determined that every drop had a charge which was a while number multiple of -1.60x10^-19. if a drop has a total charge of -9.60x10^-19 then how many excess electrons are contained within the drop?
Answers: 2

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
You know the right answer?
Questions


Mathematics, 26.02.2021 15:50

Mathematics, 26.02.2021 15:50


Mathematics, 26.02.2021 15:50



History, 26.02.2021 15:50

History, 26.02.2021 15:50


Mathematics, 26.02.2021 15:50


Mathematics, 26.02.2021 15:50

Mathematics, 26.02.2021 15:50

Mathematics, 26.02.2021 15:50


Business, 26.02.2021 15:50


Mathematics, 26.02.2021 16:00



