

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
You are given a stock solution of 500.0 mL of 1.00M magnesium chloride solution. Calculate the volum...
Questions


Mathematics, 15.11.2021 07:20



Mathematics, 15.11.2021 08:00



Mathematics, 15.11.2021 08:20

Computers and Technology, 15.11.2021 08:30

English, 15.11.2021 08:30

Mathematics, 15.11.2021 08:40



Mathematics, 15.11.2021 09:00


Mathematics, 15.11.2021 09:00




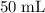 of the stock solution would be required.
of the stock solution would be required. 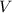 contains a solute with a concentration of
contains a solute with a concentration of  . The quantity
. The quantity  of that solute in this solution would be:
of that solute in this solution would be: 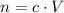 .
. . The volume of this solution is
. The volume of this solution is  . Calculate the quantity of the solute (magnesium chloride) in the required solution:
. Calculate the quantity of the solute (magnesium chloride) in the required solution: .
.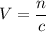 .
.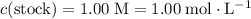 .
.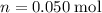 .
.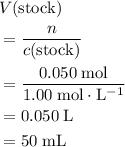 .
.

