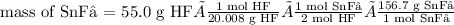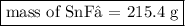Chemistry, 02.04.2021 17:40 aliceotter2007
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is prepared according
to the following equation:
Sn(s) + 2HF(aq) → SnF2(aq) + H2(g)
How many grams of tin(II) fluoride can be produced
from 55.0 g of hydrogen fluoride if there is plenty of tin
available to react?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:50
Which best describes why nh4+ can form an ionic bond with cl-?
Answers: 3


Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 07:30
Which of the following best supports the concept that genetic information is passed on to offspring from both of their parents, not just one?
Answers: 2
You know the right answer?
Tin(II) fluoride is added to some dental products to help
prevent cavities. Tin(II) fluoride is pre...
Questions

History, 07.12.2019 21:31




English, 07.12.2019 21:31

Chemistry, 07.12.2019 21:31

Mathematics, 07.12.2019 21:31




Mathematics, 07.12.2019 21:31

Mathematics, 07.12.2019 21:31



Geography, 07.12.2019 21:31

English, 07.12.2019 21:31



Computers and Technology, 07.12.2019 21:31