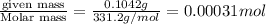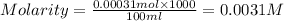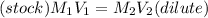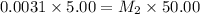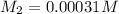Chemistry, 02.04.2021 03:00 kseniyayakimov
A stock solution was created by adding 0.1042 g of lead (II) nitrate to a 100.00 mL volumetric flask and diluting to volume with deionized water. A diluted solution was then created by removing 5.00 mL of the stock solution and placing into into a 50.00 mL volumetric flask and then diluting to volume with deionized water. What is the concentration (in molarity, M) of the diluted solution

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

You know the right answer?
A stock solution was created by adding 0.1042 g of lead (II) nitrate to a 100.00 mL volumetric flask...
Questions

Arts, 26.11.2019 14:31

Biology, 26.11.2019 14:31





Mathematics, 26.11.2019 14:31


History, 26.11.2019 14:31

Biology, 26.11.2019 14:31

Mathematics, 26.11.2019 14:31


Social Studies, 26.11.2019 14:31





Mathematics, 26.11.2019 14:31


Mathematics, 26.11.2019 14:31

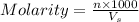
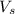 = volume of solution in ml
= volume of solution in ml
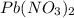 =
=