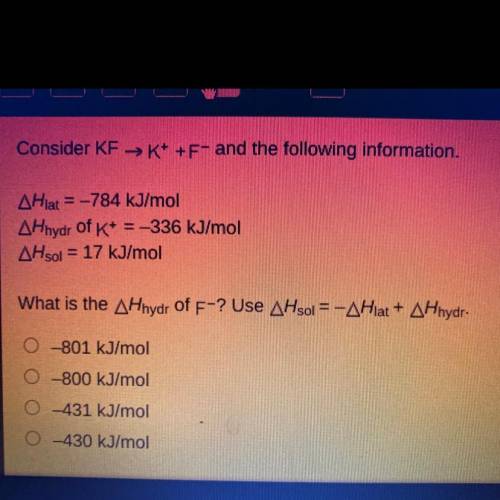
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ...

Chemistry, 02.04.2021 01:40 adelawilliams60
Consider KF →K+ +F- and the following information.
AHlat = -784 kJ/mol
AHhydr of K+ = -336 kJ/mol
AHsol = 17 kJ/mol
What is the AHhydr of F-? Use AHsol = -AHlat + AHhydr.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 15:50
Elements in group 2 are all called alkaline earth metals. what is most similar about the alkaline earth metals?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
Questions

Mathematics, 08.02.2021 21:10

English, 08.02.2021 21:20




Geography, 08.02.2021 21:20


Mathematics, 08.02.2021 21:20



History, 08.02.2021 21:20





Geography, 08.02.2021 21:20


Mathematics, 08.02.2021 21:20


Social Studies, 08.02.2021 21:20




