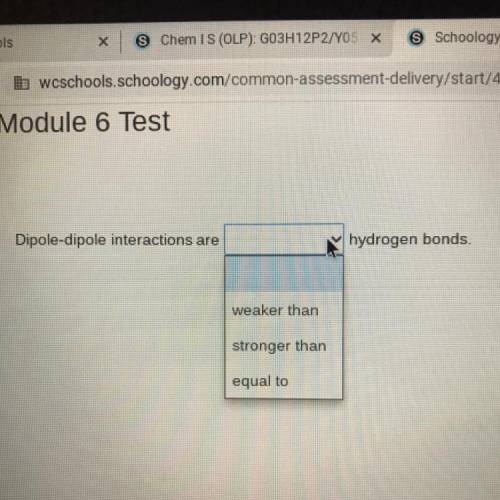
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.
...

Chemistry, 02.04.2021 01:00 postorivofarms
Dipole-dipole interactions are (weaker than, stronger than, equal to) hydrogen bonds.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2


Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
Questions



History, 31.07.2019 22:00

Social Studies, 31.07.2019 22:00


History, 31.07.2019 22:00


History, 31.07.2019 22:00


English, 31.07.2019 22:00


Social Studies, 31.07.2019 22:00

Arts, 31.07.2019 22:00



Arts, 31.07.2019 22:00

Arts, 31.07.2019 22:00





