
Chemistry, 01.04.2021 03:20 miranda911
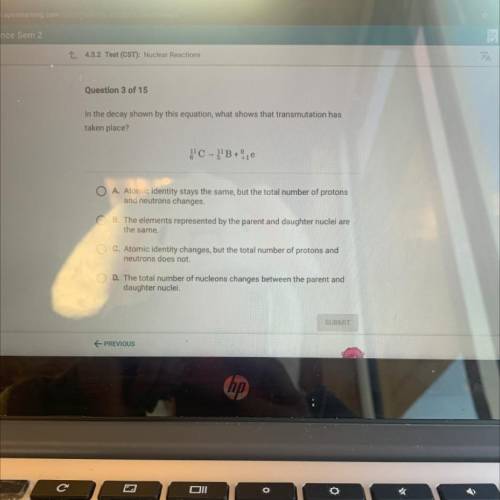
In the decay shown by this equation, what’s how’s that transmutation has taken place?
A. Atomic identity stays the same, but the total number of protons
and neutrons changes.
B. The elements represented by the parent and daughter nuclei are
the same.
C. Atomic identity changes, but the total number of protons and
neutrons does not.
D. The total number of nucleons changes between the parent and
daughter nuclei.
SUBMIT
PREVIOUS

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 2

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
In the decay shown by this equation, what’s how’s that transmutation has taken place?
A. Atomic ide...
Questions

Social Studies, 31.01.2020 06:53


Social Studies, 31.01.2020 06:53



History, 31.01.2020 06:53

Biology, 31.01.2020 06:53



Mathematics, 31.01.2020 06:53

Mathematics, 31.01.2020 06:53

Chemistry, 31.01.2020 06:53


History, 31.01.2020 06:53


English, 31.01.2020 06:53

Mathematics, 31.01.2020 06:53

History, 31.01.2020 06:53

Chemistry, 31.01.2020 06:53



