ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the m...

ANSWERED
Before you begin, keep in mind these two points:
The timer runs fast, so the minutes go faster than actual minutes.
The temperature will rise during the experiment. If the temperature gets very high, lower it to around 300 K.
Follow these steps, and then record your observations:
Locate the orange reset button on the bottom right side of the screen.
Press reset to start the reaction over.
Drag the top of the ruler upward until it reaches the 40 mark.
Drag the left platform upward until the top of the platform coincides with the 30 mark on the ruler.
Toggle the blue play/pause button to the play position at the bottom of the screen to ensure that the reaction doesn’t start before you’re ready.
Add 50 A particles, and press the play button on the bottom. Immediately start the timer using the play button on the blue box.
At every minute on the timer, pause the simulation and record the number of A and B particles.
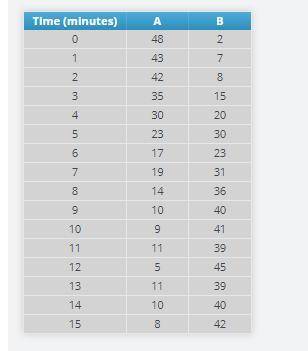

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Choose all the answers that apply. as ocean depth increases, temperature decreases temperature increases pressure increases pressure decreases salinity increases density increases
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3

Chemistry, 23.06.2019 10:00
An uncovered pot of water lies out in the sun. which statements correctly describe what happens at the surface of the liquid water? 1. the vapor pressure remains constant regardless of the water temperature. 2. the vapor pressure is produced by water molecules that have evaporated. 3. the vapor pressure increases as the sun heats the water in the pot. 4. evaporation stops once the vapor pressure reaches a certain point. 5. evaporation and condensation both occur on the liquid’s surface.
Answers: 3

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3
You know the right answer?
Questions





English, 17.12.2020 06:40




History, 17.12.2020 06:40

Advanced Placement (AP), 17.12.2020 06:40



Advanced Placement (AP), 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40




Mathematics, 17.12.2020 06:40

English, 17.12.2020 06:40



