
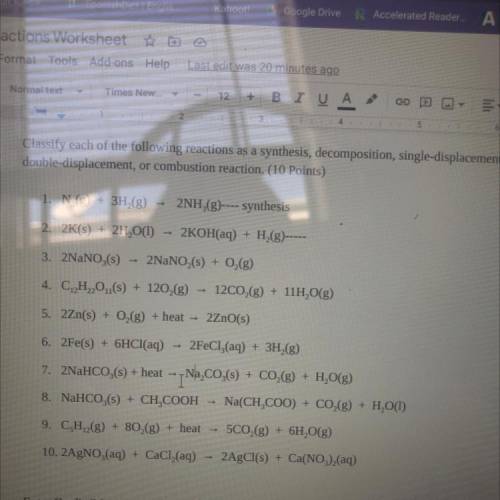
Classify each of the following reactions as a synthesis, decomposition, single-displacement,
double-displacement, or combustion reaction.
1. N2(g) + 3H2(8)
2NH (8)---synthesis
2. 2K(s) + 2H, O(1)
2KOH(aq) + H29)
3. 2NaNO (5) - 2NaNO,(s) + O2(g)
4. C, H,O,(s) + 120(8) - 12C02(g) + 11H2O(g)
5. 2Zn(s)
0,(g) + heat - 2ZnO(s)
6. 2Fe(s) + 6HCl(aq) - 2FeCl3(aq) + 3H2(8)
7. 2NaHCO,(s) + heat - Na2CO3(s) + CO2(g) + H, O(g)
8. NaHCO (s) + CH, COOH - Na(CH, COO) + CO2(g) + H, O(1)
9. CH2(g) + 80,(g) + heat - 5CO2(g) + 6H, O(g)
10. 2AgNO3(aq) + CaCl(aq) + 2AgCl(s) + Ca(NO3)2(aq)
Extra Credit (Maximum of 3 Points, 1 point each

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2
You know the right answer?
Classify each of the following reactions as a synthesis, decomposition, single-displacement,
doubl...
Questions

Biology, 30.11.2020 04:50


Advanced Placement (AP), 30.11.2020 04:50


Mathematics, 30.11.2020 04:50


Chemistry, 30.11.2020 04:50

Mathematics, 30.11.2020 04:50


Biology, 30.11.2020 04:50


Mathematics, 30.11.2020 04:50

Mathematics, 30.11.2020 04:50


History, 30.11.2020 04:50


History, 30.11.2020 04:50

Mathematics, 30.11.2020 04:50

Arts, 30.11.2020 04:50

History, 30.11.2020 04:50




