2 attempts left
Check my work
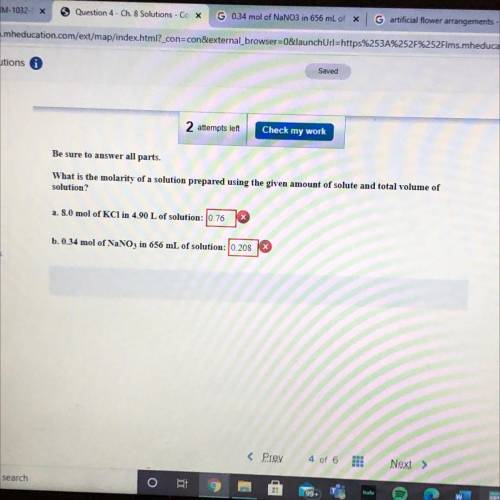
Be sure to answer all parts.
What is the molarity of a so...

Chemistry, 30.03.2021 23:30 princesstn28oqlfir
2 attempts left
Check my work
Be sure to answer all parts.
What is the molarity of a solution prepared using the given amount of solute and total volume of
solution?
a. 8.0 mol of KCl in 4.90 L of solution: 0.76
X
b. 0.34 mol of NaNO3 in 656 mL of solution: 10.208
X

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
Questions


Biology, 12.09.2019 00:10



Mathematics, 12.09.2019 00:10

History, 12.09.2019 00:10

History, 12.09.2019 00:10









Mathematics, 12.09.2019 00:10


English, 12.09.2019 00:10




