
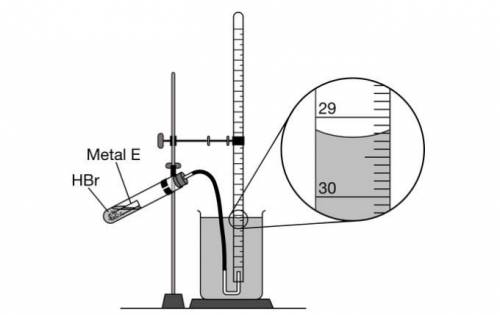
As shown in the following diagram, 10 mL samples of HBr(aq) are added to different quantities of E(s) in a closed test tube. At the start of each trial, the gas-collecting tube is filled completely with distilled water. The samples of E(s) react with HBr(aq) , and the H2(g) produced is collected in the gas-collecting tube.
The enlarged view of the gas-collecting tube at the end of trial 3 is shown in the diagram.
What should the student record as the volume, in mL , of H2(g) collected in trial 3?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The boiling point of liquids is very high what does it indicate
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

You know the right answer?
As shown in the following diagram, 10 mL samples of HBr(aq) are added to different quantities of E(s...
Questions


Mathematics, 14.07.2020 02:01

English, 14.07.2020 02:01



Mathematics, 14.07.2020 02:01


English, 14.07.2020 02:01


Geography, 14.07.2020 02:01

Computers and Technology, 14.07.2020 02:01

Biology, 14.07.2020 02:01


Mathematics, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01


Spanish, 14.07.2020 02:01

Mathematics, 14.07.2020 02:01


Social Studies, 14.07.2020 02:01



