
Chemistry, 30.03.2021 14:00 cxttiemsp021
What is the mass of 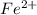 in 5 tablets of iron if the number of moles of
in 5 tablets of iron if the number of moles of 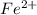 is 4.2225 x
is 4.2225 x 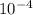 mol.
[ Ar = Fe, 56 ]
mol.
[ Ar = Fe, 56 ]

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
What is common about these molecules? a.their atoms are held together by covalent bonds. b.they are all made up of the same two atoms. c.their atoms are held together by ionic bonds. d.they are all made up of oxygen atoms only.
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 22.06.2019 16:00
How does blood clotting prevent the entry of pathogens through cuts and wounds? answer asap,, this is due tomorrow. will mark as brainliest or whatever you call it : )
Answers: 2
You know the right answer?
What is the mass of in 5 tablets of iron if the number of moles of is 4.2225 x mol.
[ Ar = Fe, 5...
Questions



Computers and Technology, 24.04.2020 15:22

Social Studies, 24.04.2020 15:22

Mathematics, 24.04.2020 15:22













Mathematics, 24.04.2020 15:22


Computers and Technology, 24.04.2020 15:22

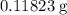 in total for the five tablets.
in total for the five tablets.  per tablet.)
per tablet.)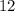 atom.
atom. 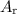 of iron is
of iron is 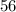 . In other words, the mass of one mole of iron
. In other words, the mass of one mole of iron 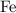 atoms would be approximately
atoms would be approximately 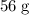 .
.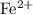 ions. Each
ions. Each 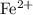 ion contains two fewer electrons than a neutral
ion contains two fewer electrons than a neutral 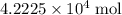 of
of 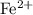 ions might be lighter than the same number of
ions might be lighter than the same number of 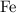 atoms by a very small extent: The mass of one mole of electrons is approximately
atoms by a very small extent: The mass of one mole of electrons is approximately 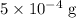 , much smaller than the mass of the same number of
, much smaller than the mass of the same number of 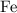 atoms (approximately
atoms (approximately 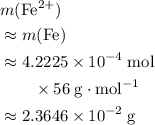 .
.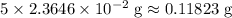 of
of 

