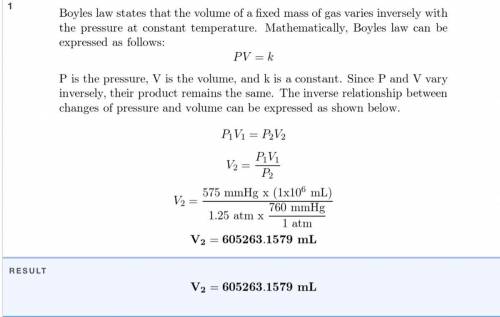
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.2...

Chemistry, 30.03.2021 09:00 hgdthbgjnb83661
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.26 atm. What will the final vol-
ume of the sample be if the temperature is
held constant?
Answer in units of mL.
help please please


Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:10
For which one of the following reactions is the value of δh° rxn equal to δh° f for the product? a. 2 h2 (g) + o2 (g) → 2 h2o (l) b. n2 (g) + o2 (g) → 2 no (g) c. 2 h2 (g) + o2 (g) → 2 h2o (g) d. h2o (l) + 1/2 o2 (g) → h2o2 (l) e. none of the above
Answers: 1

Chemistry, 21.06.2019 18:00
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 14:30
The three types is stress that act on earths rocks are compression, tension, and
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
Questions

Social Studies, 05.05.2021 18:00




History, 05.05.2021 18:00



Mathematics, 05.05.2021 18:00



Mathematics, 05.05.2021 18:00

English, 05.05.2021 18:00




Mathematics, 05.05.2021 18:00



Mathematics, 05.05.2021 18:00

Mathematics, 05.05.2021 18:00